The EndoBarrier or How You Too Can Line Your Intestines with a Trash Bag
Just saw the article on this new device today. I'm thinking with enough determination I could install a Hefty bag in my colon all by myself! :-P

Printed from: Boston Herald (http://bostonherald.com)
Device aids weight loss Saturday, March 8, 2014 -- Anonymous (not verified)
Replaces surgery by lowering blood sugars
Healthcare Sections: Sunday, March 9, 2014
Author(s):
Marie Szaniszlo
Doctors at three Massachusetts hospitals are recruiting people battling Type 2 diabetes and obesity for a clinical trial of a medical device that has been approved in other countries to reduce blood sugar and body weight without the need for the kind of weight-loss surgery that more than 200,000 Americans undergo each year.
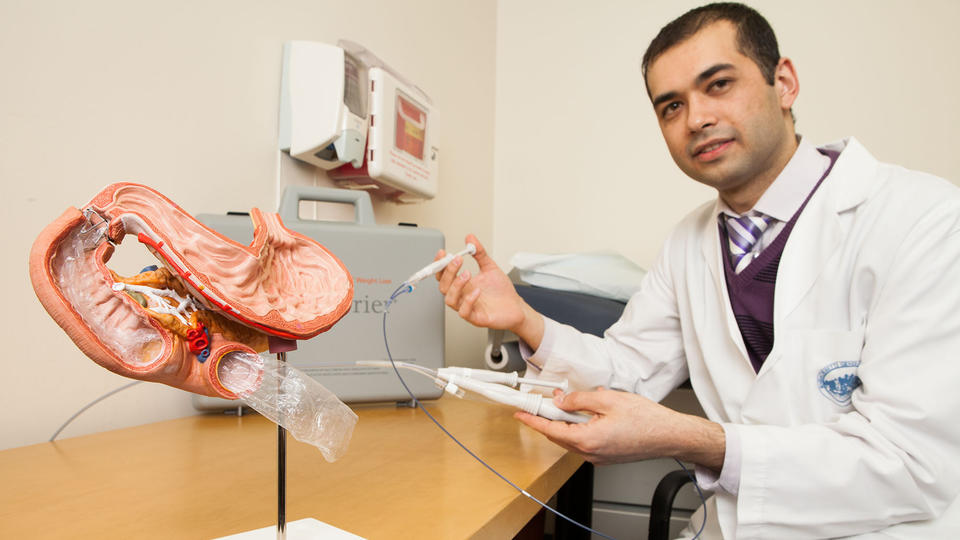
Made by Lexington-based GI Dynamics, the EndoBarrier is a thin, flexible, tube-shaped liner placed via the mouth during a brief endoscopic procedure and inserted in the duodenum, the first section of the small intestine, just beyond the stomach, said Dr. Lee M. Kaplan, the trial’s lead investigator and director of the Obesity, Metabolism and Nutrition Institute at Massachusetts General Hospital and Harvard Medical School.
“The food you eat goes down the middle of the tube,” Kaplan said, “but the tube blocks interactions between the food and hormone secretions,” which can affect insulin sensitivity, glucose metabolism, satiety and food intake.
In commercial use outside the U.S., the device has been shown to achieve as much as a 30 percent reduction in glucose levels within the first week and a 10 percent to 20 percent body-weight loss within the 12-month period for which it has been approved for use in countries including England, France, Germany and Australia, said Stuart Randle, GI Dynamics’ president and CEO.
“No one yet knows why, when you bypass the first section of the intestine, these hormones change so dramatically and so immediately,” Randle said.
The U.S. trial, which currently is enrolling people at 22 sites, including MGH, Boston Medical Center and UMass Memorial Medical Center in Worcester, will end in two years and, if it shows that the EndoBarrier is safe and effective, the Food and Drug Administration could approve the device in about a year.
If it does, the EndoBarrier could offer new hope to the 26 million people who have been diagnosed with diabetes in this country, including approximately 360,000 adults in Massachusetts, where the disease each week causes an average of 22 deaths, 38 lower-leg amputations, 13 new cases of end-stage renal disease and five new cases of blindness, according to the Massachusetts Department of Public Health.
“Obesity and diabetes are twin epidemics that remain out of control, and while we have good medical therapies for diabetes and some good therapies for obesity, they don’t always work,” Kaplan said. “For those patients who need additional therapy, this device may provide a valuable new option. But testing it is critical.”
Source URL: http://bostonherald.com/business/healthcare/2014/03/device_aids_weight_loss













0 Comments
Recommended Comments
There are no comments to display.
Create an account or sign in to comment
You need to be a member in order to leave a comment
Create an account
Sign up for a new account in our community. It's easy!
Register a new accountSign in
Already have an account? Sign in here.
Sign In Now